What Really Causes Chronic Depression?
Apr 07, 2025
What Is the Science Behind Chronic Depression?
In this blogpost, I introduce a compelling new model of depression, a model with neuroplasticity at the center. The neuroplasticity model explains much of the brain biology of depression and explains why and how various very different antidepressant treatments, including ketamine, work.
What Are the Main Causes of Depression?
For over 60 years, we have been taught that depression is due to a deficiency in the activity of a group of molecules called monoamines. These include now familiar names like serotonin, noradrenaline and dopamine. The most commonly used antidepressants, the selective serotonin reuptake inhibitors, increase the activity of these molecules. And to a modest degree, these drugs decrease some but not all of the core symptoms of depression.
Despite being so widely used and accepted, there have been niggling doubts about whether depression and other mental disorders are really caused by deficiencies or dysregulation of monoamine neuromodulators.
For the most part, these doubts or questions were pushed to the side by mainstream psychiatry, keeping the monoamine hypothesis at the forefront. Whether this was for financial gain or due to lack of a better explanation is unclear.
A note on terminology: “neurotransmitter” refers to a molecule that transmits a message from one neuron to another. “Neuromodulator” refers to molecules that modulate the impact of neurotransmitters, influence neuronal activity over a longer duration, and affect multiple neurons rather than acting at a single synapse.
As research has progressed and new, effective treatments have been discovered that don’t act on the monoamines, the doubts of a few have grown into a noisy cacophony. Below I list some of the research findings that support the need for an alternative understanding of depression (Liu, Liu, Wang, Zhang, & Li, 2017).
- Serotonin levels in the synapses (neural connections) increase within hours of taking an SSRI antidepressant like fluoxetine (Prozac®), but the clinical benefits take 4 – 8 weeks to occur. There is a disconnect here. Something other than an increase in serotonin must be responsible for the later antidepressant effects if and when they occur.
- When serotonin is depleted temporarily in healthy subjects, they don’t get depressed.
- People with genetic variants associated with low levels of serotonin are more resilient to depression than people without this variant. If the monoamine neuromodulator hypothesis were true, we would expect people with genetically determined low serotonin levels to be more vulnerable to depression rather than less.
- Antidepressant drugs that increase neuromodulator activity cause substantial improvement in only 15% more people than improve taking an inactive placebo (Stone et al., 2022). If neuromodulator dysfunction were necessary and sufficient to cause depression, one would expect better and more consistent responses.
In addition to these research findings, which increase curiosity for alternative understandings of depression, there is a strong push from patients for better treatment options. As anyone who has taken them knows, antidepressants have many unpleasant side effects, including a sense of emotional dullness and severely decreased libido and sexual function. None of these is popular.
What Is the Connection Between Stress, Depression and Neuroplasticity?
The connection between stress and depression is one of the most consistent research findings in the field. The connection has been noted well before we had molecular tools to understand how or why stress and depression were connected. Because the stress/depression connection is so strong, any plausible theory of depression needs to explain this connection. Not everyone going through a stressful time gets depressed, but experiencing repeated or chronic stressors, especially during childhood, significantly increases the risk of developing depression.
Chronic stress decreases monoamine neuromodulator levels, a finding that supports the current view of depression. But there is more, and this is where a connection between stress, neuroplasticity, and depression starts taking shape.
- Prolonged stress is associated with increased cortisol levels. That is why cortisol is often called the stress hormone.
- Prolonged elevated cortisol levels damage and kill neurons in the hippocampi, the memory centers of the brain. This decreases the ability to learn new things.
- Cortisol decreases the growth of new neuronal connections (synapses). Neuroplasticity relies on the development of new synapses. Simply put, chronic stress shuts down neuroplasticity.
- Chronic stress decreases levels of brain-derived neurotrophic factor (BDNF), one of several critical molecules required for neuroplasticity.
- Decreased neuroplasticity due to chronic stress is a big problem for someone with depression who is stuck in negative thinking and hopelessness. The decreased ability to change means that even if people engage in activities to increase their mood, it will be harder to rewire the brain circuits needed to become more resilient to stress and learn new coping mechanisms.
What Is the Science Behind Chronic Depression (the quick read)
The chronic stress/neuroplasticity model can explain many of the common signs and symptoms of depression (Price & Duman, 2020). In addition to the negative effects on the hippocampus (memory centers), chronic stress downregulates the all-important prefrontal cortex (the executive center of the brain). When the prefrontal cortex is underactive, people can’t focus and problem-solve, and the limbic system (emotional center) is given free rein to run wild with fear and pessimism.
Altered function of the hippocampal/prefrontal cortex/limbic circuit may explain why depression is associated with increased negative emotions. Other brain circuits impacted by chronic stress are associated with loss of pleasure (nucleus accumbens / lateral habenula) and helplessness (dorsal raphe). Any of you who have experienced depression will be familiar with these experiences.

How Do You Describe the Feeling of Depression?
People with depression get stuck in a rut of negative thinking. They are more likely to notice and respond to negative information and overlook or disregard the positive. This includes information relating to themselves—hence a tendency towards negative self-esteem.
The intrinsic reward pathways fueled by dopamine are underactive in depression. Therefore, people with depression don’t get the normal positive reinforcement from life and often report life as being dull and unenjoyable. In psychiatry jargon, we call this symptom anhedonia. It is anhedonia that makes life with depression so depressing. Imagine never feeling joy or happiness.
Perhaps due to this negativity bias, people with depression are more vulnerable to stress; they see stress everywhere and perceive stress as something that they can’t overcome. Cognitive functions such as attention, concentration, and memory are also impaired. All of this makes people with depression and other mental illnesses less flexible in their thinking and less able to respond adaptively to life circumstances. Focused attention is key to activating neuroplasticity.
Ketamine Therapy, a New Antidepressant Treatment
The discovery of ketamine therapy for depression has shaken up the field. Ketamine doesn’t affect monoamine neurotransmitters, yet people get better, often quickly. This has forced researchers, even reluctant ones, to consider alternative theories of what causes depression.
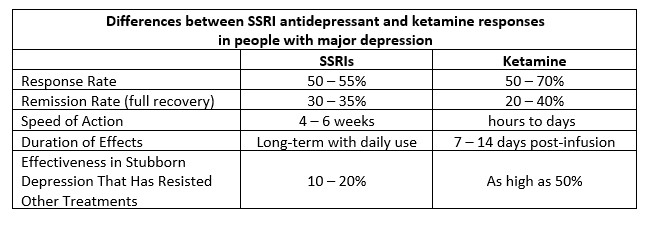
Ketamine differs from traditional antidepressants, such as specific serotonin reuptake inhibitors (SSRIs), in several ways (Cipriani et al., 2018; Jha et al., 2024; Newport et al., 2015). See the chart below for a summary.
How Does Ketamine Therapy Work? (for the science geeks among you)
Ketamine is one of a new class of drugs called psychoplastogens. These drugs cause a rapid, sharp increase in neuroplasticity. In addition to ketamine, which I describe in depth in this section, this category of drugs includes 3,4-methylenedioxymethamphetamine (MDMA), esketamine, psilocybin, and lysergic acid diethylamide (LSD).
Where legally permitted, these drugs are being used for something called medication-assisted psychotherapy. Most antidepressants are given daily to be effective. Psychoplastogens are given occasionally as a single dose. The dose creates a neuroplastic window. This, combined with skilled psychotherapy during that window in which change is more possible, allows people to see their beliefs and behaviors in a new way and get unstuck in their thinking and behavior patterns. These therapies are being used most often for depression and trauma, both of which are associated with being stuck in dysfunctional patterns.
Ketamine has two different effects, one immediate and one that acts over several days. The immediate changes can be measured at the molecular level within minutes to hours of a dose and may be related to the immediate benefits some people report. Structural changes (the creation of new neuronal connections) are observable within days. It takes time for new neural connections to grow and mature. The structural changes may be related to the longer-lasting effects of ketamine. The effects of one injection may last 1 – 2 weeks.
Here are the details (Kang, Hawken, & Vazquez, 2022). I found conflicting information about exactly how ketamine works among the papers that I read and even within papers. This suggests there is more to learn.
- Ketamine binds to and blocks the activity of N-methyl-D-aspartate (NMDA) receptors. This causes an immediate surge in glutamate throughout the brain. Changes in glutamate, glutamine, and GABA are observed soon after an injection.
- The glutaminergic receptor α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) system plays a significant role in the effects of ketamine in major depression. When AMPA is blocked, ketamine loses its antidepressant effect.
- AMPA activation leads to increases in the mammalian target of rapamycin (mTOR) and BDNF around the synapses.
- BDNF binds to its receptor, activating the downstream processes needed for neuroplasticity to occur. BDNF plays a role in the immediate molecular effects of ketamine. When BDNF is blocked, ketamine is less effective.
- mTOR mobilizes the protein reserves needed to build new neural connections over a period of days. When mTOR is blocked, ketamine is less effective.
The Ketamine—Neuroplasticity Pathway
Do All Effective Antidepressant Therapies Act on Neuroplasticity?
Once people understood that ketamine requires neuroplasticity to be effective, other treatments for depression have been studied, and, sure enough, many effective treatments have proven impacts on neuroplasticity. Some of these include the following:
- Antidepressant medications, including selective serotonin reuptake inhibitors (like fluoxetine, citalopram and others) and tricyclic antidepressants (like doxepin, amitriptyline and others). These drugs don’t work if BDNF is blocked.
- Long-term lithium treatment for bipolar disorder increases the thickness of the grey matter in the brain. Increased grey matter suggests new neural connections are being formed.
- Cognitive and behavioral therapies lead to measurable changes in the size and activity of brain regions as measured by functional imaging. Jeffrey Schwartz showed this effect in patients with obsessive-compulsive disorder as early as 1996. This finding provides evidence that psychological therapies act in biological ways.
- Electroconvulsive therapy (ECT) is a strong promoter of neuroplasticity. It increases the number of neural connections in the brain and causes measurable structural changes.
- Exercise is one of the strongest boosters of BDNF levels, neuroplasticity and mood.
- Mindfulness and meditation: Practices like meditation and mindfulness have been shown to increase gray matter volume and enhance connectivity in emotion-related brain regions, both of which are evidence of neuroplasticity.
- Emerging therapies like transcranial direct current stimulation and photobiomodulation (red light therapy) promote long-term potentiation (LTP), which is essential for learning and cognitive function, and increase BDNF levels.
This is not a comprehensive review, but it seems like many effective treatments for depression act in part through neuroplasticity.
Can Neuroplasticity Help With Depression?
In my experience, the answer to this question is a strong YES.
Over the past 10 years that I have been practicing and teaching neuroplasticity-based strategies, improved mood and decreased anxiety are among the most consistently reported improvements. What makes this striking is that few people embark on a neuroplasticity-based practice for mood symptoms. They more commonly seek relief from pain, sensitivities or fatigue. And yet, mood and anxiety often improve. This suggests that neuroplasticity-based practice is generalizable and that the effects are widespread in the brain and not only effective for certain brain pathways or regions.
Researching for this blog has led me to be more confident in recommending neuroplasticity-based practice for mental health symptoms.
What makes for an effective neuroplasticity-based practice?
- Daily practice (1 hour/day is usually recommended)
- Focused attention
- Consistent changes in thinking and behavior
- Effective calming and safety practices
- Purposeful elevation of mood
- Incremental increase in goal-directed activities
- Practice, practice, practice.
I have observed that there is no magic pill or silver bullet when it comes to improving complex chronic health conditions like depression. One must look after many aspects of health, including activity, diet, stress management, mindset and more. Neuroplasticity-based practice is a useful tool, but it won’t work in a vacuum.
And neuroplasticity-based practice takes effort. Practicing an hour a day is not most people’s idea of fun—though practice can be made fun if it is done in a creative, spontaneous way. I recommend neuroplasticity-based practice as a lifestyle over the long term. If you make changes, figure you have succeeded, and then fall back into your old patterns of thinking, feeling and behaving, your brain will revert to the structure and function that caused you problems in the first place. That’s because the brain is plastic and always able to change.

Conclusions
Now, when people ask me what causes depression and how to recover, I give a totally different answer than I did 10 years ago. I used to talk about neurotransmitters and the importance of long-term antidepressant treatment. In many cases, I still advocate these tried and tested treatments. They are useful in getting people out of the emotional black vortex they are in and providing enough relief for them to consider treatments like neuroplasticity-based practice, which require effort. Antidepressant medications and therapies like ECT can be lifesaving.
But now that I understand that neuroplasticity contributes significantly to the development of mental illness, I also understand that neuroplasticity can aid recovery. That’s another change; I now use words like “recovery” because I know that if someone can rewire their brain, they will break down the neural connections that were keeping them stuck in depression or anxiety. Neuroplasticity offers the possibility of partial or full recovery rather than being a temporary band-aid.
Mental illness is not anyone’s fault. It develops as a result of unavoidable circumstances like early childhood trauma or neglect, learned behavior, loss and social hardship. And knowledge is power. If you understand how the brain changes in response to circumstances, you can make a decision to change yours. If you change your thoughts, feelings and behaviors on a consistent basis, you will rewire your brain, and your defaults will change. Change from depression to peace is a realistic possibility.
If you are experiencing depression and would like to see whether neuroplasticity might be a strategy for you, I suggest trying my introductory course, Rewiring Your Brain For Better Health.
References
Cipriani, A., Furukawa, T. A., Salanti, G., Chaimani, A., Atkinson, L. Z., Ogawa, Y., . . . Geddes, J. R. (2018). Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. The Lancet, 391(10128), 1357-1366. doi:10.1016/S0140-6736(17)32802-7
Jha, M. K., Wilkinson, S. T., Krishnan, K., Collins, K. A., Sanacora, G., Murrough, J., . . . Anand, A. (2024). Ketamine vs Electroconvulsive Therapy for Treatment-Resistant Depression: A Secondary Analysis of a Randomized Clinical Trial. JAMA Network Open, 7(6), e2417786-e2417786. doi:10.1001/jamanetworkopen.2024.17786
Kang, M. J. Y., Hawken, E., & Vazquez, G. H. (2022). The Mechanisms Behind Rapid Antidepressant Effects of Ketamine: A Systematic Review With a Focus on Molecular Neuroplasticity. Frontiers in Psychiatry, 13. doi:10.3389/fpsyt.2022.860882
Liu, B., Liu, J., Wang, M., Zhang, Y., & Li, L. (2017). From Serotonin to Neuroplasticity: Evolvement of Theories for Major Depressive Disorder. Frontiers in Cellular Neuroscience, 11. doi:10.3389/fncel.2017.00305
Newport, D. J., Carpenter, L. L., McDonald, W. M., Potash, J. B., Tohen, M., & Nemeroff, C. B. (2015). Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression. Am J Psychiatry, 172(10), 950-966. doi:10.1176/appi.ajp.2015.15040465
Price, R. B., & Duman, R. (2020). Neuroplasticity in cognitive and psychological mechanisms of depression: an integrative model. Molecular Psychiatry, 25(3), 530-543. doi:10.1038/s41380-019-0615-x
Stone, M. B., Yaseen, Z. S., Miller, B. J., Richardville, K., Kalaria, S. N., & Kirsch, I. (2022). Response to acute monotherapy for major depressive disorder in randomized, placebo controlled trials submitted to the US Food and Drug Administration: individual participant data analysis. BMJ, 378, e067606. doi:10.1136/bmj-2021-067606